Battery Fundamentals
Batteries are electrochemical devices that store chemical energy and convert it into electrical energy. They work by using chemical reactions to move electrons from one electrode to another, creating an electrical current. This process is reversible, allowing batteries to be recharged by applying an external electrical current.
Battery Components and Functions
Batteries consist of several key components that work together to store and release energy. These components include:
- Electrodes: The electrodes are conductive materials that serve as the sites where chemical reactions occur. Batteries typically have two electrodes: an anode (negative electrode) and a cathode (positive electrode). Electrons flow from the anode to the cathode during discharge.
- Electrolyte: The electrolyte is a solution or paste that conducts ions between the electrodes. It allows the chemical reactions to occur by transporting charged particles between the anode and cathode.
- Separator: The separator is a porous material that prevents the electrodes from coming into direct contact. This prevents short circuits and ensures that the chemical reactions occur only at the electrode surfaces.
- Case: The case provides structural support and protects the internal components from the environment. It also serves as a terminal for connecting the battery to external circuits.
Battery Chemistries
Different battery chemistries utilize different materials for their electrodes and electrolytes, resulting in varying characteristics such as energy density, power output, and lifespan. Some common battery chemistries include:
- Lead-acid batteries: These batteries are commonly used in cars and other vehicles. They are relatively inexpensive and have high power output, but they are also heavy and have a limited lifespan. They consist of lead plates immersed in a sulfuric acid electrolyte.
- Lithium-ion batteries: These batteries are widely used in portable electronics, electric vehicles, and grid-scale energy storage. They offer high energy density, long lifespan, and relatively light weight. They consist of a lithium-containing compound as the anode and a transition metal oxide as the cathode, with an organic electrolyte.
- Nickel-cadmium (NiCd) batteries: These batteries were commonly used in cordless power tools and other applications, but they are being phased out due to their environmental concerns. They consist of a nickel oxide hydroxide cathode and a cadmium anode, with an alkaline electrolyte.
- Nickel-metal hydride (NiMH) batteries: These batteries offer higher energy density than NiCd batteries and are more environmentally friendly. They consist of a nickel oxide hydroxide cathode and a hydrogen-absorbing alloy anode, with an alkaline electrolyte.
Battery Types and Applications: What Is Battery
Batteries are the powerhouse of our modern world, enabling everything from powering our smartphones to propelling electric vehicles. Understanding the different types of batteries and their applications is crucial for navigating the ever-evolving landscape of energy storage.
Battery Classification Based on Usage
The primary classification of batteries is based on their usage and rechargeability.
- Primary Batteries: These are single-use batteries designed for a specific discharge cycle and cannot be recharged. They are typically cheaper than rechargeable batteries and are widely used in devices with low power requirements, such as remote controls, watches, and smoke detectors. Examples include alkaline, zinc-carbon, and lithium-iron disulfide batteries.
- Secondary Batteries: These are rechargeable batteries that can be cycled multiple times, offering a more sustainable and cost-effective solution for devices with higher power demands. They are commonly found in smartphones, laptops, electric vehicles, and grid storage systems. Examples include lead-acid, nickel-cadmium, nickel-metal hydride, lithium-ion, and flow batteries.
Battery Technologies and Their Characteristics, What is battery
Different battery technologies offer distinct advantages and disadvantages in terms of energy density, lifespan, cost, and safety.
- Lead-Acid Batteries: These are the oldest and most widely used rechargeable batteries. They are relatively inexpensive and have a high discharge rate, making them suitable for automotive applications and backup power systems. However, they have low energy density, a short lifespan, and require regular maintenance.
- Lithium-Ion Batteries: These are the most prevalent type of rechargeable batteries in consumer electronics and electric vehicles. They offer high energy density, long lifespan, and fast charging capabilities. However, they can be expensive, have safety concerns, and can degrade over time.
- Nickel-Metal Hydride Batteries: These batteries are known for their high power density and long cycle life, making them suitable for hybrid vehicles and portable power tools. They are also more environmentally friendly than lead-acid batteries but have lower energy density than lithium-ion batteries.
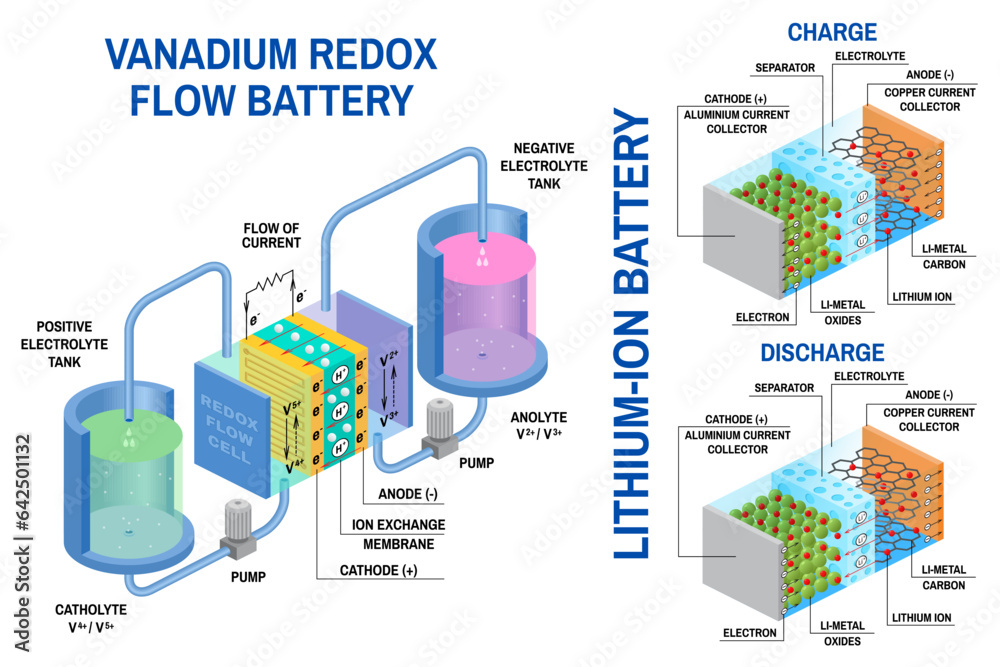
- Flow Batteries: These batteries store energy in electrolytes that are pumped through a system of cells. They are well-suited for large-scale energy storage applications, such as grid-level storage, due to their long lifespan and high discharge rates. However, they are expensive and have a lower energy density compared to other battery types.
Battery Applications Across Industries
Batteries play a pivotal role in various industries, enabling energy storage and powering devices with diverse functionalities.
- Consumer Electronics: Lithium-ion batteries power smartphones, laptops, tablets, and other portable devices, providing the convenience of wireless operation and extended usage time.
- Electric Vehicles: Lithium-ion batteries are the primary energy source for electric vehicles, offering a zero-emission alternative to gasoline-powered vehicles. Battery technology advancements are constantly driving range and performance improvements in electric vehicles.
- Grid Storage: Batteries are increasingly used for grid-scale energy storage, helping to stabilize the power grid and improve the reliability of renewable energy sources. Flow batteries and lithium-ion batteries are commonly employed in grid storage applications.
- Medical Devices: Batteries power implantable medical devices such as pacemakers, defibrillators, and insulin pumps, enabling life-saving medical treatments and improving patient outcomes.
- Aerospace and Defense: Batteries are used in aircraft, satellites, and military equipment to provide power for communication, navigation, and other critical functions. Lithium-ion batteries are particularly favored for their high energy density and lightweight design.
Battery Performance and Safety

Understanding the performance and safety aspects of batteries is crucial for their effective utilization and to ensure safe operation. Battery performance is characterized by factors such as capacity, voltage, and current, while safety concerns include overheating, leakage, and explosion.
Battery Capacity, Voltage, and Current
Battery capacity, voltage, and current are fundamental parameters that define a battery’s performance and suitability for specific applications.
* Battery Capacity represents the amount of electrical charge a battery can store. It is typically measured in ampere-hours (Ah) or milliampere-hours (mAh). A higher capacity battery can deliver power for a longer duration.
* Battery Voltage refers to the electrical potential difference between the battery’s positive and negative terminals. It is measured in volts (V). The voltage determines the power output of the battery.
* Battery Current is the rate at which electrical charge flows through the battery. It is measured in amperes (A). The current determines the amount of power delivered by the battery at a given time.
For instance, a 12V, 100Ah battery can deliver 12 volts of potential difference and can provide 100 amperes of current for one hour, or 10 amperes for 10 hours, or 1 ampere for 100 hours.
Factors Affecting Battery Performance
Several factors can influence the performance of a battery, including temperature, discharge rate, and cycle life.
* Temperature significantly affects battery performance. High temperatures can lead to faster degradation and reduced capacity, while extremely low temperatures can hinder the chemical reactions within the battery, resulting in lower power output.
* Discharge Rate refers to the rate at which a battery is discharged. A higher discharge rate can lead to increased internal resistance and reduced capacity.
* Cycle Life represents the number of charge-discharge cycles a battery can endure before its capacity significantly deteriorates. Batteries with a longer cycle life are more suitable for applications requiring frequent charging and discharging.
For example, lithium-ion batteries used in electric vehicles typically have a cycle life of 500-1000 cycles, meaning they can be charged and discharged 500-1000 times before their capacity drops to 80%.
Safety Concerns Related to Batteries
Batteries can pose safety risks if not handled properly. Some common safety concerns include overheating, leakage, and explosion.
* Overheating can occur due to excessive charging or discharging, high ambient temperatures, or internal short circuits. Overheating can lead to battery damage, reduced performance, and even fire hazards.
* Leakage can occur when the battery’s internal components break down, releasing electrolytes or other corrosive materials. Leakage can damage surrounding components and pose a health risk.
* Explosion is a rare but serious risk associated with batteries, particularly with lithium-ion batteries. Explosions can be triggered by overheating, physical damage, or improper handling.
To mitigate these risks, it is essential to use batteries from reputable manufacturers, follow the manufacturer’s instructions for charging and discharging, and avoid exposing batteries to extreme temperatures or physical damage.
What is battery – The term “battery” carries a dual meaning, encompassing both the physical device that powers our electronic world and the legal concept of unlawful physical contact. While the former fuels our smartphones and laptops, the latter evokes the power of music, as exemplified by the electrifying performances of Star Jackson , a renowned guitarist who ignites stages with her raw talent.
The energy she generates through her music is a testament to the potent force of artistic expression, a force that, like a battery, can both illuminate and inspire.
A battery, in its simplest form, is a device that stores chemical energy and converts it into electrical energy. This concept of stored energy, much like the potential for discourse, is amplified when we consider the online sphere, particularly in spaces like skai jackson reddit , where discussions about a public figure like Skai Jackson can spark debates and generate a dynamic energy of their own.
The potential for this energy, whether it be chemical in a battery or social in online discourse, is what makes both fascinating subjects of study.